https://www.meritnation.com/ask-answer/question/distinguish-between-conductors-and-insulators-give-three-exa/physics/3158266
- 1
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminium, the movable charged particles are electrons.Insulators are non-conducting materials with few mobile charges and which support only insignificant electric currents.
An electrical insulator is a material whose internal elctric current do not flow freely, and which therefore does not conduct an electric current, under the influence of an electric current.
- 0
- 0
Conductors and Insulators
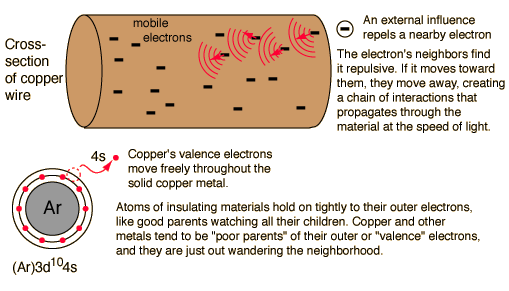
In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, having extremely high resistance to the flow of charge through them. "Conductor" implies that the outer electrons of the atoms are loosely bound and free to move through the material. Most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly repel each other. Any external influence which moves one of them will cause a repulsion of other electrons which propagates, "domino fashion" through the conductor.
Simply stated, most metals are good electrical conductors, most nonmetals are not. Metals are also generally good heat conductors while nonmetals are not.
- 2
Conductors and Insulators

In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, having extremely high resistance to the flow of charge through them. "Conductor" implies that the outer electrons of the atoms are loosely bound and free to move through the material. Most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly repel each other. Any external influence which moves one of them will cause a repulsion of other electrons which propagates, "domino fashion" through the conductor.
Simply stated, most metals are good electrical conductors, most nonmetals are not. Metals are also generally good heat conductors while nonmetals are not
- 0
Conductors and Insulators

In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, having extremely high resistance to the flow of charge through them. "Conductor" implies that the outer electrons of the atoms are loosely bound and free to move through the material. Most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly repel each other. Any external influence which moves one of them will cause a repulsion of other electrons which propagates, "domino fashion" through the conductor.
Simply stated, most metals are good electrical conductors, most nonmetals are not. Metals are also generally good heat conductors while nonmetals are not
- 0
Hello Srajan,
Conductors and Insulators

In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, having extremely high resistance to the flow of charge through them. "Conductor" implies that the outer electrons of the atoms are loosely bound and free to move through the material. Most atoms hold on to their electrons tightly and are insulators. In copper, the valence electrons are essentially free and strongly repel each other. Any external influence which moves one of them will cause a repulsion of other electrons which propagates, "domino fashion" through the conductor.
Simply stated, most metals are good electrical conductors, most nonmetals are not. Metals are also generally good heat conductors while nonmetals are not.
All the best in your studies
- 2
Conductors and Insulators

i hope you have understanded it ! ! !
soooooo cheersssssss ! ! ! ! ! ! ! !
- 0

